Abstract
4-Vinylcyclohexene diepoxide (VCD), an occupational chemical that targets ovarian follicles and accelerates ovarian failure in rodents, was used to test the effect of early-onset reproductive senescence on mammary fibroadenoma formation. One-month female Sprague Dawley rats were dosed with VCD (80 mg/kg or 160 mg/kg) and monitored for 22 months for persistent estrus and tumor development. Only high-dose VCD treatment accelerated the onset of persistent estrus relative to controls. However, both doses of VCD accelerated mammary tumor onset by 5 months, increasing incidence to 84% (vs. 38% in controls). Tumor development was independent of time in persistent estrus, 17β-estradiol, androstenedione and prolactin. Delay in VCD administration until after completion of mammary epithelial differentiation (3 months) did not alter tumor formation despite acceleration of ovarian senescence. VCD administration to 1-month rats acutely decreased mammary alveolar bud number and expression of β-casein, suggesting that VCD’s tumorigenic effect requires exposure during mammary epithelial differentiation.
1. Introduction
Fibroadenomas are the most common form of benign breast disease, occurring in 25% of women [1]. In addition to being associated with an increased risk of breast cancer, mammary fibroadenomas are also of clinical importance as these benign tumors account for up to 50% of all breast biopsies [1]. However, little is known about the precise pathogenesis of mammary fibroadenomas; thus further exploration of their etiology is warranted.
Spontaneous mammary tumors occur with varying incidence in most strains of rats, including Sprague Dawley (SD) rats. The majority are benign fibroadenomas, with only 18% of these being malignant [2,3]. Although 70% of female SD rats ultimately develop mammary tumors over their ~24 month life span [4], tumor onset does not typically occur before post-natal day (PND) 400 [5,6]. The initiation and/or progression of mammary tumors in SD rats is hormone-dependent as ovariectomy with or without adrenalectomy between PND 77–102 almost completely prevents mammary tumor development, reducing the overall incidence from 70% to 4% [4,7]. Tumor onset in rats typically occurs concurrently with the onset of acyclicity, which in rats, unlike in women, is marked by a transient phase characterized by persistent secretion of 17β-estradiol from existing follicles (persistent estrus) [8,9]. Because of this temporal association, a causal role for 17β-estradiol’s direct or indirect effects (e.g. inducing lactrotrope hypertrophy and prolactin secretion) on mammary tumor development during this transitional period has been proposed, but not proven [10]. However, no additional information regarding the hormone-responsiveness of these spontaneous tumors is available.
In order to elucidate specific elements of the hormonal milieu that impact fibroadenoma formation in female SD rats, we chose to examine the effect of an ovary-intact, chemically-induced model of premature ovarian failure on mammary tumor occurrence. The occupational chemical 4-vinylcyclohexene diepoxide (VCD), produced in the plastics industry and studied by the National Toxicology Program in the 1980’s [11–16], has been exploited in translational research after its effects on the female reproductive system were discovered [10–14]. In this chemical model of pre-mature ovarian failure, repeated administration of VCD to one-month rodents can accelerate the natural process of pre-antral folliclular atresia within the ovary by inhibition of c-KIT survival signaling [13]. In the absence of a pool of immature follicles, the onset of ovarian failure is accelerated [12–16]. Thus, one might initially postulate that this chemically-accelerated form of ovarian failure could limit mammary tumor development, analogous to the protective effects of surgically–induced ovarian failure (ovariectomy). However, a careful comparison of the hormonal changes occurring in each of these ovarian failure models suggests alternative hypotheses. Firstly, because androgens suppress mammary tumor formation [17], the persistence of ovarian androgen production in ovary-intact, VCD-induced ovarian failure (vs. complete ablation of the ovary with ovariectomy) may prevent the occurrence of spontaneous mammary tumors. Alternatively, by accelerating the onset and/or prolonging the duration of persistent estrus during the transition to ovarian failure, VCD-treatment could actually speed mammary tumor development. To assess the relative importance of these alterations in ovarian hormone production on fibroadenoma development, experiments were undertaken to assess the dose-dependent effects of VCD on spontaneous mammary tumor development during persistent estrus and the onset of ovarian senescence in SD rats.
2. Materials and methods
2.1 Animals procedures
Young (1-month) female Sprague Dawley rats (Harlan Laboratories) were housed in plastic cages and maintained on a 12L/12D schedule at 22 ±2 °C, with food (Teklad Global Diet 2018S) and water available ad libitum. After one week of acclimation to the animal facility, rats were randomly assigned to treatment groups. 4-Vinylcyclohexene diepoxide (VCD; ≥ 96% purity) was obtained from Sigma and stored at −20 °C. Following established protocols [16], young SD rats were administered 25 intraperitoneal (ip) doses of VCD between post-natal days (PND) 35–68 (80 mg/kg or 160 mg/kg; n = 12 and 21, respectively), or vehicle (1.25 µL/g/d DMSO; n = 17). VCD dosages were selected based on their known ovotoxic effects in the Fischer F344 rat strain [16]. A subset of animals from vehicle (n = 7) and high dose VCD (160 mg/kg; n = 10) treatment groups was sacrificed following dosing on PND 53 in order to assess direct effects of VCD on the developing breast. The remaining animals were monitored visually and by palpation for mammary tumor onset and incidence by individuals blinded to treatment group for 570 days post-treatment, which was equivalent to PND 604, or 22 months of age, where 1 month = 28 days). In order to determine whether the stage of mammary development at time of VCD exposure affected outcome, a separate study was conducted in which mature (3-month) SD rats were treated with the same VCD doses (80 mg/kg or 160 mg/kg × 25 d; n = 7 or n = 12, respectively) or vehicle (n = 17) between PND 94–119 and monitored for mammary tumor onset and incidence for 261 days post-treatment, which was equivalent to PND 355, or 13 months of age. Morning blood serum and plasma samples were collected periodically throughout the experiment from the tail vein in anesthetized rats (8 mL xylazine, 5 mL ketamine, 2 mL acepromazine; 1 µL/g), and stored at −80 °C for subsequent hormone assays. All experiments were approved by The University of Arizona IACUC and conformed to the Guide for the Care and Use of Experimental Animals.
2.2 Mammary tumor histopathology
Mammary tumors were removed at the termination of the experiment or were surgically excised during the experimental period from anesthetized animals if tumors reached a size that restricted the animal’s mobility (~3 cm diameter). Animals with excised tumors remained in the study and were monitored for tumor formation at new sites. Mammary tumors were fixed in phosphate buffered formalin, and transferred to 70% ethanol after 48 hours. Tumors were then paraffin embedded, and 5 µm sections were stained with hematoxylin and eosin for histologic evaluation. Tumor histopathology was determined by an ACVP board-certified veterinary pathologist (DGB) who was blinded to treatment groups.
2.3 Hormone assays
Circulating 17β-estradiol and androstenedione were measured using commercially available radioimmunoassays (Siemens) as per manufacturer’s protocol with sensitivities of 2.5 pg/mL and 0.03 ng/mL, respectively. Plasma prolactin levels were measured by enzyme-linked immunosorbent assay (ELISA, Calbiotech) with a sensitivity of 0.2 ng/ml.
2.4 Evaluation of estrous cyclicity
Temporal changes in reproductive status were monitored each month for 10 day intervals by assessment of vaginal cytology based on standard techniques [8,9]. Briefly, vaginal smears were considered indicative of proestrus if round, nucleated epithelial cells were observed, estrus if large cornified cells were observed, metestrus if a combination of leukocytes, cornified and round epithelial cells were observed, and diestrus if round epithelial cells and leukocytes were observed. As previously described [8], the reproductive stage of each animal was further classified as “epithelial phase” when only nucleated or cornified cells were present in the vaginal smear, and the beginning of ovarian senescence was considered to be ≥ 75% of days in the epithelial phase, which is indicative of persistent estrus and precedes ovarian failure, defined here as the end of persistent estrus [8,9].
2.5 Mammary whole mounts analysis
The fourth mammary gland, harvested on PND 53 after administration of 15 doses of 160 mg/kg/d VCD or vehicle to 1-month rats (n = 7 per group) was mounted on slides and fixed overnight in 1:3 acetic acid:ethanol, followed by de-fatting in acetone for 10 days. Acetone was refreshed every 48 hours. Tissues were then stained overnight with carmine alum, dehydrated in a series of graded ethanol, and stored in glycerol. The distal 5 mm margin of the mammary whole mount was examined for terminal end buds (TEB), terminal ducts (TD) and alveolar buds (AB; 3–5 buds), by light microscopy. Epithelial structures were determined using the criteria of Russo and Russo [18] by an individual blinded to treatment groups. Briefly, a bulb-shaped terminal ductal structure > 100 µm in diameter with three to six epithelial cell layers in the perimeter of the bulb was designated a terminal end bud (TEB). A terminal duct (TD) was classified as a terminal structure < 100 µm in size with one to three epithelial layers between the ductal lumen and the outer edge of the structure. Alveolar buds (ABs) were lobular structures composed of three to five buds in one cluster. Alveolar bud lobules (> 5 buds) were not detected in the distal 5 mm margin at the time point examined.
2.6 Real-time quantitative RT-PCR
RNA was isolated from mammary tissue of vehicle-treated or VCD-treated rats (160 mg/kg/d; n = 7 and n = 10, respectively) on PND 53 after 15 doses. Mammary tissue was harvested, flash-frozen in liquid nitrogen and stored at −80 °C. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) and followed by 2.5M lithium chloride precipitation, as previously described [19]. RNA (250 µg) was reverse transcribed (iScript; BioRad) and changes in expression of c-KIT (Rn00573942_m1), KIT-ligand (Rn01502851_m1), cyclin D1 (Rn00432359_m1) and β-casein (Rn01524626_m1) were determined by TaqMan real-time RT-PCR analysis in using rat-specific primers obtained from Applied Biosystems. Data were analyzed using the comparative cycle threshold (C1) method as a means of relative quantitation of gene expression, normalized to an endogenous reference (18s RNA), as previously described [19].
2.7 Immunofluorescence and confocal microscopy
The fourth mammary glands, isolated from 53 day-old rats treated with 15 daily doses of vehicle vs. 160 mg/kg/d VCD, were fixed in phosphate buffered formalin, paraffin embedded, sectioned (5 µm), deparaffinized, and prepared for confocal microscopy, as previously described [13]. Briefly, sections were treated for antigen retrieval by immersion in citrate buffer (10 mM sodium citrate; pH 6.0) and microwave heating for 2.5 minutes at boiling temperature, followed by 7 minutes at sub-boiling temperature. Tissue sections were blocked with 5% BSA/PBS, and polyclonal rabbit antihuman c-KIT (1:400 dilution; A4502, Dako), which is known to react with rat c-KIT [20], was applied for 18 h, followed by biotinylated goat anti-rabbit secondary antibody (Vector) at a 1:75 dilution for 1 h (4 °C) with further streptavidin/biotin amplification. Sections were treated with Cy5-streptavidin (Jackson ImmunoResearch Labs), followed by the nuclear stain YOYO-1 (Molecular Probes). Slides were washed with PBS, coverslipped with aqueous mounting medium, and stored in the dark at 4 °C until viewed on Zeiss (LSM 510 NLOMeta) confocal microscope with an argon and helium-neon laser projected though the tissue into a photomultiplier at λ = 633 and 488 nm for Cy5 and YOYO-1, respectively. Specificity of primary antibody for detection of c-KIT was determined by verification of the absence of staining when primary antibody was omitted (data not shown).
2.8 Statistical methods
Kaplan Meier survival plots were analyzed by Logrank Test with post-hoc Logrank Test for trend to confirm dose-dependency of tumor development. Chi-square analysis was performed to compare tumor incidence between VCD- and vehicle-treated groups at individual time points. One-way analysis of variance (ANOVA) with Bonferroni post-hoc testing, repeated measures one-way ANOVA for serial hormone analysis, or student unpaired t-test was performed on parametric data, as appropriate. All analyses were performed with InStat software (Graphpad, San Diego, CA).
3. Results
3.1 Ovarian Function Following PND 35–68 VCD Administration
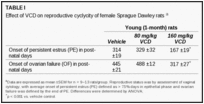
The onset of persistent estrus in low dose VCD (80 mg/kg)-treated animals was no different than vehicle-treated rats (PND 329 ±32 vs PND 314 ±19, p > 0.05; Table 1). In contrast, administration of high dose VCD significantly accelerated the onset of persistent estrus (PND 167 ±19, p < 0.001 vs. vehicle; Table 1). Similarly, the transition from persistent estrus to ovarian failure was unchanged in rats treated with low dose VCD and was accelerated in high dose VCD-treated rats (PND 317 ±27 vs. 445 ±21 in vehicle, p < 0.001; Table 1). Circulating levels of 17β-estradiol and androstenedione, assayed at 90 day intervals, were not different in VCD-treated animals as compared to controls, including at the end of the experiment (Table 2).
3.2 Mammary Tumor Onset and Incidence Following PND 35–68 VCD Administration
In control (vehicle-treated) animals, as anticipated from prior reports, mammary tumors were not clinically evident until PND 394 (Figure 1A). At this time point, all control rats had been in a state of persistent estrus for approximately 2.5 months (Table 1; Figure 1C). In contrast, mammary tumors were evident as early as PND 219 in both high and low dose VCD-treated animals, preceding tumor onset in vehicle-treated controls by 6 months (175 days; Figure 1A). In rats treated with high dose VCD, tumor onset occurred less than 2-months after the accelerated onset of persistent estrus, while tumor onset in low dose VCD rats actually preceded persistent estrus by 4 months (110 days; Figure 1A,C). Mammary tumor incidence over time was higher in VCD-treated rats vs. vehicle-treated controls (p = 0.03) and was dose-dependent (p < 0.01; Figure 1A). Final tumor incidence in both low and high dose VCD animals significantly exceeded that of controls (83–85% vs. 38%; Figure 1A). Additionally, VCD had a dose-dependent effect on tumor burden, as the average number of tumors per affected rat was double and triple that of controls with 80 and 160 mg/kg VCD, respectively (Figure 1B).
3.3 Effect of Delayed (PND 94–119) VCD Administration on Ovarian Function and Mammary Tumors
As initial data suggested that the accelerated onset and higher incidence of mammary tumors in VCD-treated rats were not closely related to the onset or duration of persistent estrus, mature 3-month SD rats were dosed with VCD to assess the importance of mammary developmental stage at time of VCD exposure. Effects of VCD on ovarian function in 3-month animals recapitulated those demonstrated in 1-month rats, as low dose VCD did not alter the onset of persistent estrus, while the onset of persistent estrus in high dose VCD-treated animals was accelerated as compared to controls (PND 290 ±8.2 vs. PND 341 ±12, p < 0.01). However, treatment with either dose of VCD in mature animals did not increase mammary tumor incidence relative to control animals (tumor incidence at PND 355 was 0% in all 3 groups). When normalized to time post-VCD treatment (261 days), tumor incidence in young VCD-treated animals was significantly increased (38%; PND 295) relative to VCD-treated mature animals (0%, p < 0.01; PND 355), suggesting that tumor onset was dependent on age at time of VCD exposure.
3.4 Mammary Gland Histopathology
On gross inspection and palpation, all mammary tumors in vehicle and VCD-treated animals were subcutaneous, moderately firm and freely movable. A subset of tumors excised from the animals was examined histologically (27%, n = 14 of 52). All tumors were benign, with histopathology consistent with fibroadenoma for all specimens (Figure 2A), with the exception of one papillary cystadenoma (Figure 2B). The fibroadenomas (Figure 2A) were characterized as non-encapsulated, well-demarcated masses composed of multiple lobules of dense fibrous connective tissue separating numerous small ductules lined by low cuboidal epithelium. Cells within the connective tissue were spindle shaped with plump round to ovoid vesiculate nuclei, while ductular epithelial cells had minimal cytoplasm and round nuclei with finely stippled chromatin and occasional multiple nucleoli. The mitotic index was 0–2/HPF. The papillary cystadenoma (Figure 2B) was a partially encapsulated, well-demarcated mass composed of multiple lobules composed of cysts and intercalated papillary projections lined by a single layer of cuboidal to low columnar epithelium. Scant fibrous stroma was present, although there were a few small foci of dense fibrous tissue randomly interspersed in the mass. Epithelial cells had minimal cytoplasm and round to oval nuclei with finely stippled chromatin and occasional multiple nucleoli. Mitotic figures were rare. The cysts often contained hemorrhage with occasional hemoglobin crystals and clusters of hemosiderin-laden macrophages.
3.5 Prolactin Assay
Because spontaneous fibroadenomas in SD rats have been postulated to occur in response to elevated prolactin during persistant estrus [21], serum prolactin levels were compared between groups at multiple times throughout the experimental period. Prolactin levels, while tending to increase with age in all groups, were not different between VCD-treated and control animals at any time point during the study (Figure 3).
3.6 Mammary Whole Mount Analysis During VCD Exposure
As VCD only stimulated fibroadenoma formation when exposure occurred during a time of rapid mammary gland proliferation and differentiation, direct effects of VCD (160 mg/kg/d, PND 28–53) vs. vehicle on mammary ductal epithelial structures were assessed (Figure 4). The number of terminal end buds (TEB) and terminal ducts (TD) were not significantly different between vehicle- and VCD-treated animals (Figure 4A,B); however, the number of alveolar buds (AB) was significantly decreased (−36%) with VCD dosing (p = 0.02; Figure 4A–D).
3.7 Direct Effect of VCD on Gene Expression of Markers of Mammary Gland Proliferation and Differentiation
Expression of cyclin D1, a proliferation marker for the mammary epithelium [20], was not changed with VCD treatment (160 mg/kg/d, PND 28–53; Figure 5A), whereas, gene expression of β-casein, a milk protein and biomarker for differentiation and maturation of the mammary epithelium [22], was down-regulated with VCD exposure (Figure 5A). Expression of c-KIT and KIT-ligand, genes associated with cell growth and differentiation in the mammary gland [23], was next assessed in mammary ductal epithelium since VCD is known to target c-KIT signaling in ovarian follicles [13]. c-KIT and KIT-ligand genes were expressed in control mammary tissue, but their expression levels were not significantly changed following 15-days of VCD treatment (160 mg/kg/d) in PND 53 rats (Figure 5A). Consistent with this finding, immunofluorescent c-KIT protein, which localized to mammary ductal epithelial structures in control tissue (PND 53, Figure 5B,D), was not noticeably different with respect to expression pattern or intensity in VCD-treated vs. control animals (Figure 5C,E).
4. Discussion
Benign mammary fibroadenomas have been linked to increased risk of breast cancer, indicating that, while not necessarily causative, benign and malignant breast lesions may share a common underlying mechanism or risk factor. Pre-malignant stages of mammary tumor development can best be manipulated and studied in rodent models, and these models may ultimately shed light on the etiology of human disease. Mammary tumor development has been linked to a number of risk factors, both exogenous (e.g. environmental exposure) and endogenous (e.g. sex hormones) [24,25]. The ovotoxic chemical 4-vinylcyclohexene diepoxide (VCD) was selected in this study due to its ability to manipulate the time of onset of ovarian failure by accelerating the normal process of follicular atresia in rats [16]. The initial aim of this study was to elucidate the specific effect of accelerated onset of persistent estrus, as well as ovary-intact, androgen-replete ovarian failure on spontaneous mammary tumor development.
VCD treatment, when begun at 1 month of age, did in fact alter mammary tumor development, accelerating the onset and increasing the incidence of mammary tumors, with a latency period of 6 months (185 days) relative to onset of exposure. Observed differences in mammary tumor onset and incidence, however, could not be attributed to VCD-induced changes in ovarian function, as mammary tumors appeared in 1-month VCD-treated animals even before changes in ovarian function and achieved a higher incidence despite a similar duration of persistent estrus in VCD-treated vs. control animals. Moreover, high dose VCD treatment of 3-month old rats, while accelerating the onset of persistent estrus, did not alter the onset or incidence of mammary tumor development. Thus, these results suggest that, contrary to our initial hypotheses, VCD’s tumorigenic effect may be mediated via direct effects on the mammary gland, and that early onset of ovarian senescence was not a factor in stimulating tumor onset or incidence in this model.
The absence of a role for ovarian hormones in mediating VCD-induced mammary tumor formation was further confirmed by assessment of ambient serum levels of 17β-estradiol and androstenedione in VCD-treated rats, which were no different than controls at any time. Further, because rising prolactin (PRL) levels in a setting of elevated follicle stimulating hormone have also been postulated to drive mammary tumor formation in normal rats [21], serum PRL levels were compared between groups. While tending to increase with age in all animals, PRL levels were not different between VCD and control animals at any time point during the study, suggesting that prolactin was not a causative factor driving the high tumor incidence seen in VCD-treated animals.
While finding no evidence for a hormonal cause of increased tumor formation in VCD-treated rats, the evidence instead pointed to a possible direct tumorigenic effect of VCD on the mammary epithelium, as tumor incidence only increased when VCD exposure occurred during the period from PND 35–68 when terminal end buds proliferate and differentiate into alveolar buds [18]. It would thus appear that VCD can be included in the growing list of polycyclic hydrocarbons, such as 7,12 dimethylbenz [α] anthracene (DMBA), that induce mammary neoplasia by targeting the developing mammary ductal system during this developmental stage, classically known as “the window of susceptibility” [18,26,27]. DMBA, which stimulates proliferation of undifferentiated mammary epithelium during TEB development, is frequently used experimentally to induce mammary carcinoma formation [22,26–31]. In contrast to DMBA, VCD appeared to target mammary epithelial differentiation, reducing mammary alveolar bud number and expression of β-casein milk protein, a biomarker for mature mammary epithelium [22], while having no effect on proliferation (i.e. TEB number and cyclin D1 expression). Thus, en toto, these data suggest that blockade of mammary epithelial differentiation may induce fibroadenoma formation, while enhanced proliferation of undifferentiated epithelium drives carcinoma formation. Chemical disregulation of epithelial proliferation vs. differentiation may thus have an impact on the type of epithelial transformation (malignant vs. benign) that develops during the lifespan of an animal. Accelerated tumor formation with VCD treatment could also be a species- and strain-specific effect, as Sprague Dawley rats are highly prone to spontaneous fibroadenoma development with age [4–6].
The c-KIT signaling pathway is a key regulator of cell differentiation and survival in a wide range of tissues [32–34]. While its role in mammary epithelium has not been a focus of study, it is known that loss of c-KIT expression is associated with malignant transformation of mammary epithelia in both rodents and humans [35–37]. Because abrogation of c-KIT signaling by VCD and decreased c-KIT gene expression is the primary driver of VCD-induced ovotoxicity [13], we investigated the possibility that disruption of the c-KIT pathway in mammary tissue by VCD during epithelial differentiation could be permissive for later tumor development. However, neither c-KIT nor its ligand was significantly altered by VCD treatment on PND 53 after 15 days of VCD treatment. Although it remains possible that c-KIT expression was altered at other time points in the study, mammary c-KIT expression may be more highly correlated with malignant than benign transformation [20], potentially explaining why changes in c-KIT were not detected. It should be noted that these data are the first, to our knowledge, to document expression and localization of c-KIT, an understudied mammary signaling pathway, in the mammary ductal epithelium of female Sprague Dawley rats.
In conclusion, VCD-induced mammary tumorigenesis may be a useful tool for examining perturbations in the physiology of branching morphogenesis in the breast, as well as the pathophysiology of fibroadenomas, a common disorder in women that is associated with increased breast cancer risk and is responsible for the majority of mammary biopsies [1]. Because human mammary fibroadenoma is an understudied disease, this novel model may provide new insights into the underlying cellular and molecular mechanisms responsible for mammary neoplasia.
Acknowledgements
This work was supported by the National Institutes of Health [grant number 5R21AT003614].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.